GUIDELINE for Feline Leishmaniosis
The Leishmaniosis in cats guidelines were first published in the J Feline Med Surg 2013, 15: 638-642 by Maria Grazia Pennisi et al. The present guidelines were updated by Maria Grazia Pennisi.
Key points
- Leishmaniosis is less well known in cats than in dogs and humans; felids were traditionally considered to be resistant to Leishmania infection, whereas canids are the main reservoir.
- Cats are infected by the same Leishmaniaspecies that infect dogs and humans in tropical and subtropical areas worldwide.
- Competent sand fly vectors take blood meals from cats and become infected when feeding on cats with infantum.
- Only sporadic feline disease cases have been reported worldwide in canine leishmaniosis endemic areas, mainly caused by infantum.
- Epidemiological investigations have confirmed, however, that feline infections are not rare and disease occurrence might be underestimated in endemic areas.
- More than half of cases show dermatological and mucocutaneous lesions (mainly ulcerative and nodular dermatitis) and lymph node enlargement; sometimes these are the only findings at physical examination.
- Ocular (blepharoconjunctivitis, keratitis, uveitis, panophthalmitis) and oral (nodules, chronic gingivo-stomatitis) lesions, as well as general clinical signs (weight loss, decreased appetite, and lethargy), anaemia, hyperglobulinaemia, and proteinuria are reported in 20-30% of cats.
- Many other clinical manifestations are sporadically reported and a causative role for infantum has not always been demonstrated and often coinfections and comorbidities are seen.
- Parasitological confirmation of leishmaniosis can be obtained by cytology, histology with immunohistochemistry, culture or by PCR, performed on samples taken from the skin, mucous membranes, lymph nodes, bone marrow, blood or any other affected tissues.
- Antibody testing using different techniques has been used to assess infection prevalence and to support the diagnosis in suspected clinical cases.
- Therapy is empirically based on the same drugs used to treat dogs affected by canine leishmaniosis but they are not licenced for treating cats. Allopurinol (sometimes in combination with meglumine antimoniate) is the most frequently used drug.
- Clinical cure can be obtained but the infection is not cleared, and recurrence of clinical signs can occur after cessation of therapy, similarly to dogs.
- Prognosis is influenced by comorbities (neoplasia, chronic kidney disease) and these have to be carefully investigated.
- Prevention based on the application of pyrethroid ectoparasiticides which are effective against sand fly bites is possible with flumethrin collars. Any other pyrethroids are not licenced for cats and very toxic.
Agent properties
After malaria and lymphatic filariasis, leishmaniosis is the third most important vector-borne disease in people; it is caused by protozoan parasites of several Leishmania species, most of zoonotic concern (Solano-Gallego et al., 2009). In veterinary medicine, canine leishmaniosis caused by Leishmania infantum (CanL) is of primary interest, because dogs are the main reservoir of the parasite for humans, and also because management of the canine disease is often challenging (Baneth et al., 2008; Miró et al., 2017). However, the epidemiology of leishmaniosis is complex and vectorial transmission can involve different host species as reservoirs (Maroli et al., 2007; Gonzales et al., 2017; Cardoso et al., 2021).
Cats are found to be naturally infected by the same Leishmania species detected in dogs and humans in tropical and subtropical areas worldwide and L. infantum is the most reported species in cats from the Mediterranean basin, Iran and Brazil (Hatam et al., 2009; Vides et al., 2011; Pennisi et al., 2015a; Attipa et al., 2017a; Mohebali et al., 2018; Akhtardanesh et al., 2020; Asgari et al., 2020; Baneth et al., 2020; Costa-Val et al., 2020; Fernandez-Gallego et al., 2020). Feline infections with dermotropic species include, in the Old World, Leishmania tropica in Turkey and Iran and Leishmania major in Turkey (Paşa et al., 2015; Can et al., 2016; Akhtardanesh et al., 2020), while in the New World they are caused by Leishmania mexicana in Texas (USA) and Venezuela (Trainor et al., 2010; Rivas et al., 2018; Paniz Mondolfi et al., 2019), Leishmania braziliensis in Brazil and French Guiana (Schubach et al., 2004; Rougeron et al., 2011; Costa-Val et al., 2020), Leishmania amazonensis in Brazil (de Souza et al., 2005; Carneiro et al., 2020) and Leishmania venezuelensis in Venezuela (Bonfante-Garrido et al., 2001).
Epidemiology
Leishmaniosis caused by L. infantum is a globally emerging disease (Baneth et al., 2008; Solano-Gallego et al., 2009). Using molecular diagnostic techniques on dogs in endemic areas, it was shown that the prevalence of the infection is much higher than that of the disease manifestation (Lombardo et al., 2012). The same is true for people: in some endemic areas, latent infections in 30% of the population were estimated, based on a positive Leishmanin Skin Test, LST (Kaplan et al., 2009). Feline leishmaniosis (FeL) is sporadically reported worldwide, usually from the same areas where the disease occurs in dogs or humans (Pennisi et al., 2015a). In the Old World, feline L. infantum infection has been detected in Mediterranean countries (Italy, Spain, Portugal, France, Greece, Turkey, Cyprus) and Iran and characterized feline isolates are similar to those obtained from dogs or humans (Pennisi and Persichetti, 2018). Cases have been reported in Switzerland, in cats imported from Spain and increased pet movement and rehoming throughout Europe will likely lead to more disease reporting also in non-endemic areas (Rüfenacht et al., 2005; Richter et al., 2014).
Prevalence
Epidemiological studies have been increasingly performed over the last 20 years in endemic areas using tests that detect antibodies (immunofluorescence [IFA], ELISA, Western blot [WB], direct agglutination [DA] tests) and tests that detect parasite (cytology, immunohistochemistry [IHC], PCR). The rates of infection vary widely, which could be due to the methodology used, the geographic area, and the population under study. Positive blood PCR rates range between 0.43 and 61% of the cat population tested, and antibody prevalence between 3 and 59% with very few studies finding zero positivity rates (Pennisi et al., 2015a). However, percentage of positivity is usually lower compared to dogs in the same areas (Maia et al., 2010; Otranto et al., 2017).
A systematic review with meta-analysis recently examined 78 articles that reported antibody and PCR prevalences and estimated a 10% (95% CI: 8%-14%) overall prevalence in feline populations worlwide. Italy was the country with a highest antibody (24%) and PCR (21%) prevalence, compared to other countries (Asfaram et al., 2019).
It has however to be mentioned that a peculiarity of L. infantum epidemiology is a patchy spread with endemic or hyperendemic foci within areas with lower prevalence or the absence of infected vectors or hosts (Gradoni, 2018; Baneth et al., 2020).
Transmission
Leishmania spp. are vector-borne protozoan parasites transmitted by phlebotomine sand fly vectors to vertebrate hosts (Fig. 1).

Fig. 1. Life cycle of Leishmania donovani. Wikipedia, public domain.
Amastigotes replicate in intracellular vacuoles within macrophages of vertebrate hosts and are ingested by haematophagous female sand flies during the blood meal. In the sand fly gut amastigotes change to flagellated extracellular promastigote forms and replicate. Then the infected sand fly transmits the promastigates to a new vertebrate host as promastigotes are regurgitated during the blood meal. There is no evidence that ticks and fleas are involved in natural Leishmania transmission (Baneth et al., 2008; Solano-Gallego et al., 2009). There is a risk for transmission in the absence of sand flies as transplacental infection has been demonstrated in dogs, humans and wild animals (Baneth et al., 2008; Solano-Gallego et al., 2009; Martín-Sánchez et al., 2020). Venereal transmission is a potential route of infection (da Silva et al., 2009). Other types of direct transmission have been suggested, but interestingly mainly vertical transmission is considered responsible for the high incidence and prevalence in hunting dogs in the USA, in the absence of sand fly vectors (Baneth et al., 2008; Solano-Gallego et al., 2009; Toepp et al., 2017). The risk of transmission by blood transfusion is important for humans and dogs in endemic areas (Baneth et al., 2008; Solano-Gallego et al., 2009; Kaplan et al., 2009) and it potentially can occur also in cats, as healthy cats are frequently found positive, when testing EDTA-blood samples with PCR or even with cytological evaluation of blood smears (Persichetti et al., 2016; Attipa et al., 2017a; Brianti et al., 2017; Diakou et al., 2017; Metzdorf et al., 2017; Otranto et al., 2017).
Many studies have demonstrated that sand flies take blood meals from cats (Gonzáles et al., 2017) and they have been found infected by L. infantum after feeding on naturally infected cats (Maroli et al., 2007; da Silva et al., 2010; Mendonça et al., 2020). Experimental xenodiagnoses (In case of vector-borne infections, xenodiagnoses is a diagnostic method used to document the presence of infectious agents by exposing a host to a vector and then examining the vector for the presence of the infectious agent it may have ingested.) by sand flies between a cat naturally infected with L. infantum and a naïve dog was successfully performed (Batista et al., 2020). Additionally, antibodies against sand fly salivary antigens were detected in cats exposed to phlebotomine sand flies in an endemic area and the feline antibody response to Phlebotomus perniciosus saliva was positively associated with Leishmania infection (Pereira et al., 2019a).
Pathogenesis
Progressive infection and disease development are primarily influenced by immunological factors, which are linked to the complex genetic background of the hosts, as demonstrated by studies in laboratory animals, dogs and humans (Baneth et al., 2008). In people, L. infantum induces two different diseases: cutaneous leishmaniosis is generally a focal papular or ulcerative skin lesion whereas visceral leishmaniosis is a severe disease without cutaneous manifestations. The latter form is the typical presentation in immunocompromised patients (Kaplan et al., 2009). In dogs, leishmaniosis is a multisystemic disease with a wide and dynamic spectrum of severity that reflects the balance between the protective cell-mediated, and the non-protective humoral immune response. Usually, subclinical infection and self-limiting mild disease forms outnumber non-self-limiting severe disease cases in endemic areas (Baneth et al., 2008; Solano-Gallego et al., 2009). Coinfections with vector-borne pathogens (primarily Ehrlichia canis) influence parasite burden and the progression of CanL (Baxarias et al., 2018).
In all host species, macrophages play a central role in the control of the infection. Cytokines such as IFN-γ, IL-2 and TNF-α, secreted by activated T cells, stimulate macrophages to kill intracellular Leishmania amastigotes. The presence of Leishmania-infected macrophages is usually associated with a granulomatous inflammatory reaction (Baneth et al., 2008). Lymphoid hyperplasia is the most common cytologycal pattern in both dogs and cats (Solano-Gallego et al., 2009, 2011; Pennisi et al., 2015a). A massive antibody response is associated with more severe disease and is responsible for immune complex deposition in the kidney, with glomerulonephritis and subsequent chronic kidney disease (CKD) (Baneth et al., 2008; Solano-Gallego et al., 2009).
Many data from experimental infections of dogs are available but experimental infections of cats with L. infantum were performed in only two studies. An additional limitation is that these two studies followed up infected cats for only 16 (Akhtardanesh et al., 2018) or up to 24 (Kirkpatrick et al., 1984) weeks. Kirkpatrick et al. (1984) detected seroconversion in cats from week two p.i., while cats intraperitoneally infected by Akhtardanesh et al. (2018) were not antibody-positive even at week 16. Clinical or laboratory signs were not seen in these two studies, apart from reduced packed cell volume and hyperproteinaemia observed by Akhtardanesh et al. (2018). Parasitaemia was investigated by culture (Kirkpatrick et al., 1984) and PCR (Akhtardanesh et al., 2018). Kirkpatrick et al. (1984) used two different strains for their infections and parasites were cultured on weeks one and two only with one strain. Blood PCR was positive throughout the study by Akhtardanesh et al. (2018). Kirkpatrick et al. (1984) cultivated amastigotes from liver and spleen by post-mortem evaluations of infected cats and they also found amastigotes on cytological evaluations of spleen, liver, bone marrow and blood smears. Conversely, amastigotes were not found on histopathology of lymph nodes, bone marrow, spleen or liver by Akhtardanesh et al. (2018), but moderate sinus histiocytosis was seen in two cats (Akhtardanesh et al., 2018). Findings from these two studies suggest that cats are more resistant than dogs to experimental infection; however, chronic infection cannot be excluded. This is what has been inferred from a previous longitudinal study on naturally infected cats that remained PCR- and antibody-positive for a long time with either no clinical manifestations or with a mild disease, and from cats diagnosed with FeL years after rehoming from an endemic area (Pennisi et al., 2015a).
The description of histopathological patterns associated with FeL is available mostly from biopsied lesions from skin, mucosal lesions and the eye. Feline skin lesions show a diffuse granulomatous dermatitis with Leishmania parasites inside macrophages, or a granulomatous perifolliculitis and lichenoid tissue reaction/interface dermatitis, with a lower parasite load (Navarro et al., 2010; Puleio et al., 2011). Granulomatous inflammation with many amastigotes has also been seen in mucosal nodules, eye, spleen, liver, and the kidneys (Navarro et al., 2010; Puleio et al., 2011; Verneuil, 2013; Migliazzo et al., 2014).
Immunity
Leishmania immunity is complex. In the dog, a susceptible species, protective immunity against L. infantum is CD4 T cell-mediated, and the release of IFN-γ, IL-2 and TNF-α is associated with anti-Leishmania activation of macrophages. A combination of high antibody levels and a reduced cell-mediated response are found in dogs developing disease (Baneth et al., 2008; Solano-Gallego et al., 2009).
Cats with L. infantum associated clinical disease have high blood parasitaemia, low to very high antibody levels and hyperglobulinaemia. As reported for dogs, Leishmania PCR-positive healthy cats can be negative for anti-Leishmania antibodies (Pennisi et al., 2012, 2015a). It was recently shown that, as in other hosts, both humoral and cell-mediated adaptive immune responses are elicited by L. infantum in exposed cats from endemic areas. Specific IFN-γ was in fact detected in 21% of these cats and they all were negative on Leishmania PCR performed on EDTA blood samples and negative or border-line antibody-positive (Priolo et al., 2019). However, the relationship between the pattern of adaptive immune response and the severity of FeL has not yet been investigated.
Infection experiments with L. braziliensis have shown that the development of skin lesions usually precedes antibody appearance, and seroconversion occurs when the lesions are healing (Simoes-Mattos et al., 2005).
Many cats that developed clinical signs were suspected to have an impaired immune system because of concurrent FIV infection, dual FIV and FeLV infection, neoplasia (squamous cell carcinoma), diabetes mellitus, immune-mediated diseases (e.g., eosinophilic granuloma, pemphigus foliaceus, feline chronic stomatitis) or had been treated with immunosuppressive drugs such as corticosteroids (Pennisi and Persichetti, 2018; Pennisi et al., 2019; Fernandez-Gallego et al., 2020). However, specific IFN-γ production has been detected in both FIV- and FeLV-coinfected cats, including a FeLV-infected cat with concurrent squamous cell carcinoma (Priolo et al., 2019).
Clinical signs
Canine leishmaniosis has been extensively investigated by field cross sectional and longitudinal controlled studies. It is usually a chronic and progressive disease, with an incubation period lasting for months or even years. Any tissue or organ can be involved, but skin lesions are most suggestive, and renal disease is most important prognostically for the reduced survival time (Solano-Gallego et al., 2009; Geisweid et al., 2012; Roura et al., 2020).
Clinical cases of FeL have been documented – mainly in the last 25 years and in Southern Europe – and case reports and case series at present provide the only published information about the disease. This means that the current level of evidence for statements and recommendations about FeL management is lower compared to CanL. Feline leishmaniosis is probably underreported and less frequent and the less severe clinical presentations are probably missed, as occurred previously with CanL. Additionally, coinfections and comorbidities are commonly seen in cats with FeL and these can contribute to a misrepresentation of disease (Pennisi et al., 2015a).
The age range of cats at diagnosis is wide (2-21 years) but FeL is more common in mature animals (median age seven years). Only very few cases are diagnosed in 2-3 year old cats. Both genders are similarly represented and almost all cases are seen in domestic shorthair cats (Pennisi et al., 2015a; Maia et al., 2015; Migliazzo et al., 2014; Basso et al., 2016; Pennisi et al., 2016; Attipa et al., 2017b; Leal et al., 2018; Franchi et al., 2019; Pennisi et al., 2019; Pereira et al., 2019b; Fernadez-Galleo et al., 2020).
Based on the prevalence of clinical manifestations in reported cases, frequent (found in over 50% of cases), common (affecting 25-50% of cats) and rare (seen in less than 25% of cats) clinical signs and clinico-pathological abnormalities should be differentiated (Pennisi et al., 2015a).
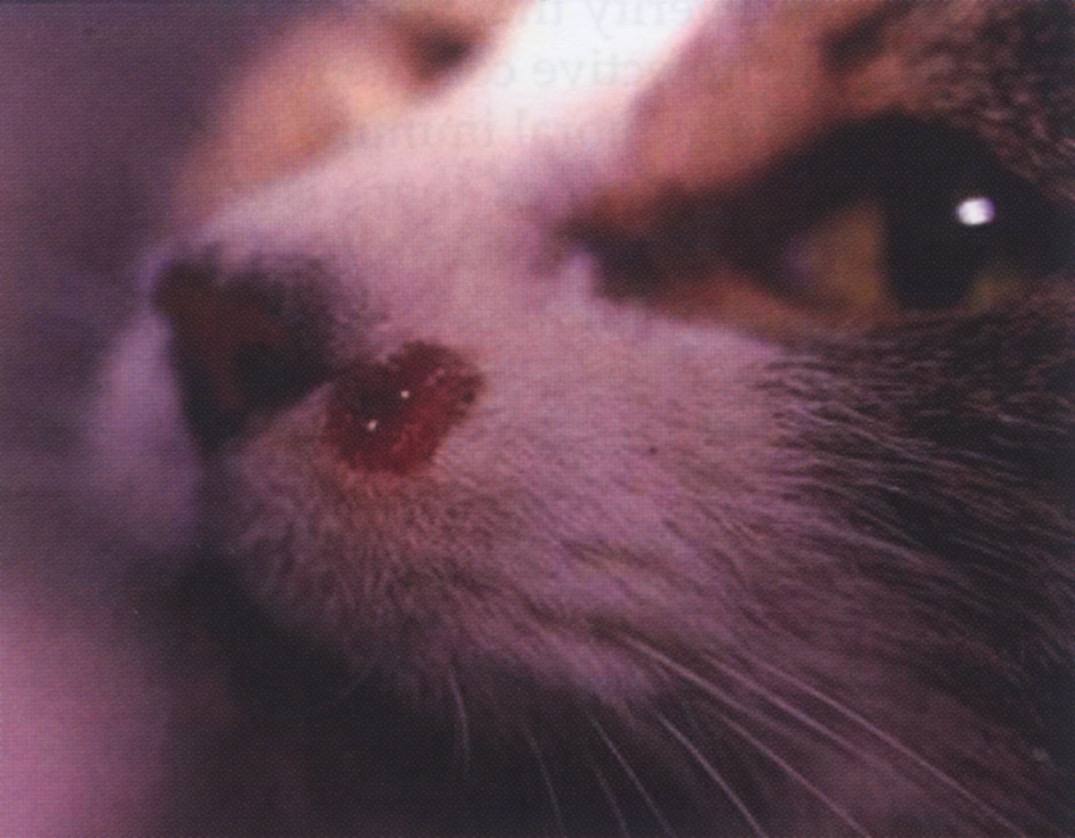
Skin and muco-cutaneous lesions are the most frequently reported manifestations seen in more than 60% of cases but rarely they are the only abnormality found. The most frequent skin lesion is focal and multifocal crusty-ulcerative dermatitis (Pennisi et al., 2015b; Basso et al., 2016; Fernadez-Galleo et al., 2020) (Fig. 2).

Fig. 2. Leishmania infantum associated ulcerative dermatitis in a cat (courtesy Maria Grazia Pennisi)
Large lesions with raised margins are seen symmetrically on distal limbs (carpus) and pressure points (hock, ischial tuberosity) as in dogs, but smaller lesions or diffuse ulcerative dermatitis can be observed on the head, trunk and footpads. Focal, multifocal and diffuse nodular dermatitis is also frequent. Nodules are usually small (less than 1 cm in diameter) and they are firm, alopecic and non-painful, rarely ulcerated, mainly distributed on the head and muco-cutaneous junctions (Pennisi et al., 2015a; Basso et al., 2016; Attipa et al., 2017b; Leal et al., 2018; Pereira et al., 2019b; Fernandez-Gallego et al., 2020). Ulcerative and nodular lesions can be seen at the same time in cats (Pennisi et al., 2016; Basso et al., 2016). In contrast to dogs, diffuse scaly dermatitis and alopecia are not common findings in cats (Ozon et al., 1998; Navarro et al., 2010; Fernandez-Gallego et al., 2020). Haemorrhagic bulla with amastigotes detected on cytological evaluation in two cats is a skin lesion that has never been reported in canine leishmaniosis (Pennisi et al., 2004) (Fig. 3).

Fig. 3. Leishmania infantum associated hemorrhagic nodule in a cat (courtesy Maria Grazia Pennisi)
Pruritus is usually not reported apart from cases with concurrent pruritic skin disease such as flea allergy, eosinophilic granuloma, pemphigus foliaceus, and demodicosis (Pennisi et al., 2015a; Leal et al., 2018).
Mild to severe lymph node enlargement is seen in about half of cases and a multicentric pattern is most common (Pennisi et al., 2015a).
Among the common clinical signs, non-specific manifestations such as lethargy, anorexia and weight loss predominate (Pennisi et al., 2015a; Pennisi et al., 2019; Fernandez-Gallego et al., 2020). About one third of cats show ocular involvement with eyelid, conjunctival, corneal, uveal, and retinal lesions. The progression of diffuse uveal granulomatous inflammations leads to panophthalmitis (Migliazzo et al., 2014; Pennisi et al., 2015a; Pimenta et al., 2015; Fernandez-Gallego et al., 2020).
Oral disease includes gingival ulcerations, nodular glossitis, epulid-like lesions, chronic stomatitis and faucitis, and it is observed in about 20% FeL cases (Poli et al., 2002; Pennisi et al., 2004; Leiva et al., 2005; Maroli et al., 2007; Ibba, 2009; Migliazzo et al., 2014; Pimenta et al., 2015; Pennisi et al., 2016; Fernandez-Gallego et al., 2020). In some cats cytological, histological and PCR investigations were performed and the parasite was found in the inflamed oral tissue (Migliazzo et al., 2014; Fernandez-Gallego et al., 2020).
Chronic upper respiratory tract disease with amastigotes found in the nasal discharge has been rarely reported (Ibba, 2009; Migliazzo et al., 2014). A severe obstructive syndrome was seen in three cats which was associated to nodular or infiltrative masses formed by granulomatous leishmanial inflammation in the nasal cavity and the rhinopharynx (Leal et al., 2018; Franchi et al., 2019; Altuzarra et al., 2020).
Dehydration, pale mucous membranes, vomiting, hepatomegaly, fever, jaundice, polyuria/polydipsia, spleen enlargement, abortion and mastitis form a list of rare findings and a causative role for L. infantum has not always been demonstrated (Pennisi et al., 2015a; Pereira et al., 2019b).
Laboratory and diagnostic imaging findings
Laboratory changes
Unfortunately, thorough clinico-pathological evaluations in cats with FeL have not always been performed. Non-regenerative anaemia, hyperglobulinaemia, elevated alfa- and gammaglobulin fractions, and proteinuria are the most frequently reported abnormalities (Pennisi et al., 2015a; Fernandez-Gallego et al., 2020; Urbani et al., 2020; Savioli et al., 2021). As in canine leishmaniosis, lymphoid hyperplasia is the lymph node cytological pattern seen in lymph node fine-needle aspirate smears taken from infected cats (Perillo et al., 2013).
In a few cases followed up until death or euthanasia, CKD developed and it was the more common cause of death (Pennisi et al., 2016; Hartmann et al., 2020).
Diagnostic imaging
In three cats with severe obstructive syndrome in the upper respiratory tract caused by L. infantum, computed tomography (CT) of the head (Franchi et al., 2019; Altuzarra et al., 2020) and rhinoscopy (Leal et al., 2018; Altuzarra et al., 2020) were used to evaluate anatomical abnormalities and to take guided biopsies of nodular or infiltrative lesions. In one very severe case the osteolytic damage involved even the ethmoidal cribriform plate and the nasal mass extended into the rostral cranial cavity affecting the olfactory bulbs (Altuzarra et al., 2020). Neoplastic diseases and fungal rhinitis are the main conditions that can show similar imaging patterns.
Diagnosis
Diagnosis can be confirmed by direct parasitological (cytology, histology with IHC, PCR or culture) and indirect antibody tests (IFA, ELISA, DA, and WB). In most feline cases in which cytology, culture and PCR were performed, a diagnosis was confirmed from lymph node, skin, bone marrow or blood samples (Fig. 4).

Fig. 4. Lymph node smear from a cat: Leishmania amastigotes in a macrophage (MG-G stain; x64 objective). Photomicrograph courtesy Maria Grazia Pennisi)
In some cases ocular disease caused by L. infantum was diagnosed by histology after enucleation of the affected eye. One study estimated the prevalence of FeL in 2,632 feline skin and ocular biopsies examined over a four-year period to be 0.57% (Navarro et al., 2010). Rhinoscopy-guided biopsies were used for histological diagnosis (Leal et al., 2018; Franchi et al., 2019; Altuzarra et al., 2020). Upon post-mortem examination, the parasite was identified also from samples of the spleen, liver, kidney, pancreas, and gastro-intestinal tract (Pennisi et al., 2015a). Nodular or diffuse granulomatous leishmanial inflammation was observed in affected tissues.Immunohistochemistry is recommended for increasing the sensitivity of histopathology in CanL (Solano-Gallego et al., 2009; Navarro et al., 2010; Puleio et al., 2011).
Antibody tests for leishmaniosis are not commercially available but IFA tests have been validated for usage on feline samples with a cut off set at 1:80 dilution (Pennisi et al., 2012; Persichetti et al., 2017; Iatta et al., 2020). When the diagnostic performance of IFA, ELISA and WB was compared, all three techniques were reliable, but WB was the most accurate (Persichetti et al., 2017). Rapid tests on the market for the qualitative detection of canine anti-L. infantum antibodies are not validated for use with cat serum. When one of these commercial rapid tests was compared to an IFA validated assay, low levels of sensitivity, negative predictive values, specificity, and positive predictive values were observed (Pennisi et al., 2017).
Treatment
The available information is based on single case reports or case series of FeL caused by L. infantum, not always with an appropriate follow-up (EBM grade IV) (Pennisi et al., 2015a). Treatment of cats with clinical leishmaniosis is still empirically based and off label, using the most common drugs administered to dogs. Therefore, cats under therapy should be carefully monitored for possible adverse effects (table 1). Long-term administration of allopurinol (5-50 mg/kg SID or BID) is the most used drug regimen and usually clinically effective, even in FIV-infected cats (Pennisi et al., 2015a; Maia et al., 2015; Pennisi et al., 2019; Altuzarra et al., 2020; Fernandez-Gallego et al., 2020), but some cases do not respond to treatment with any administered drug (Pennisi et al., 2015a; Fernandez-Gallego et al., 2020). Additionally, L. infantum infection is not cleared and clinical signs can recur after stopping therapy, as is the case in dogs (Pocholle et al., 2012; Pennisi et al., 2015a; Pennisi et al., 2016; Attipa et al., 2017b; Fernandez-Gallego et al., 2020). In a few cases allopurinol was given as maintenance therapy after a course of meglumine antimoniate (usually 50 mg/kg SID SC) (Basso et al., 2016; Pereira et al., 2019b; Fernandez-Gallego et al., 2020) and in one case with miltefosine (Fernandez-Gallego et al., 2020) or domperidone (Dedola et al., 2015) respectively. Domperidone or miltefosine were given as a second line drug in one case each (Maia et al., 2015; Leal et al., 2018). However, it has to be mentioned that propylene glycol is among the excipients of the miltefosine oral formulation licensed for the treatment of CanL. Propylene glycol can cause Heinz body formation as demonstrated in a pilot study on healthy cats treated with miltefosine for 15 days (Bouchez et al., 2019). A consequence of Heinz body formation is a decreased life span of feline red blood cells. Surgical excision of nodules was performed in two cats, but this was followed by recurrence (Costa Durao et al., 1994; Rüfenacht et al., 2005). In table 2 dosages and duration of treatments used in FeL, as well as the total numbers of cats treated and those clinically improved are reported.
Table 1: Reported adverse drug reactions in cats treated for feline leishmaniosis
| TREATMENT | ADVERSE DRUG REACTION (number of cases) |
BIBLIOGRAPHY |
|---|---|---|
| ALLOPURINOL | Increased liver enzymes (1) | Rüfenacht et al., 2005 |
| AKI after some weeks (2) | Pennisi et al., 2016 | |
| Head and neck dermatitis (1) | Leal et al., 2018* | |
| Pruritus (1) | Brianti et al., 2019 | |
| MEGLUMINE ANTIMONIATE and ALLOPURINOL | AKI and death in 5 days (1) | Fernandez-Gallego et al., 2020 |
| MEGLUMINE ANTIMONIATE | AKI at day 25 (1) | Leal et al., 2018* |
| MILTEFOSINE | Worsened azotaemia (1) | Leal et al., 2018* |
Legend: AKI= acute kidney injury; * a single cat had to be switched to different drugs due to adverse drug reactions or lack of efficacy.
Table 2: Treatments used in clinical cases of feline leishmaniosis and reported clinical efficacy with numbers of clinically improved cats out of those treated
| TREATMENT | DOSAGE | DURATION and NOTES | BIBLIOGRAPHY (number of cats clinically improved / total treated) |
|
|---|---|---|---|---|
| ALLOPURINOL | 5-50 mg/kg | PO q12-24h | 1.5-36 months Surgical excision of nodules associated (3 cats) Silver nitrate cauterization of an ulcer (1 cat) |
Rüfenacht et al., 2005 (2/2) Leiva et al., 2005 (1/1) Sanches et al., 2011 (1/1) Pocholle et al., 2012 (1/1) Filecci I., 2012 (1/1) Richter et al., 2014 (1/1) Migliazzo et al., 2014 (1/1) Pimenta et al., 2015 (1/1) Basso et al., 2016 (0/1)^ Pennisi et al., 2016 (3/6) # Attipa et al., 2017b (1/1) Altuzarra et al., 2020 (1/1) Leal et al., 2018 (0/1)* Brianti et al., 2019 (1/1) Franchi et al. 2019 (0/1)^ Pereira et al., 2019b (1/1)** Fernandez-Gallego et al., 2020 (5/7)** |
| MEGLUMINE ANTIMONIATE and ALLOPURINOL |
MA: 50 mg/kg | SC q24h | 30 days | Pimenta et al., 2015 (2/2) Basso et al., 2016 (1/1)^ Pereira et al., 2019b (1/1)** Fernandez-Gallego et al., 2020 (2/3) |
| ALL: 10 mg/kg | PO q12-24h | long-term maintenance treatment | ||
| Surgical treatment of large skin ulcers (1 cat) | ||||
| MEGLUMINE ANTIMONIATE |
50 mg/kg | SC q24h | 20-40 days | Costa Duraõ et al., 1994 (1/1) Ibba, 2009 (1/1) Leal et al., 2018 (1/1)* Franchi et al., 2019 (0/1)^ Fernandez-Gallego et al., 2020 (1/2) |
| 375 mg/cat | SC q48h | 55 days | ||
| 300 mg/cat | SC q24h | 4 months | ||
| MEGLUMINE ANTIMONIATE and KETOCONAZOLE |
MA: 5 mg/kg | SC q24h | Both given for 3 cycles of 4 weeks duration, 10 days apart | Hervás et al., 1999 (1/1) |
| KCZ: 10 mg/kg | PO q24h | |||
| DOMPERIDONE and ALLOPURINOL |
DOM: 0.5 mg/kg | PO q24h | 2 courses of 28 days, 6 months apart | Dedola et al.,2015 (1/1) |
| ALL: 10 mg/kg | PO q12h | 12 months | ||
| MILTEFOSINE | 2 mg/kg | PO q24h | 30 days | Leal et al., 2018 (1/1)* |
| NUCLEOTIDES and ACTIVE HEXOSE CORRELATED COMPOUNDS |
Nu: 292.5 mg/cat | PO q24h | Long-term maintenance treatment | Leal et al., 2018 (0/1)* |
| AHCC: 157.5 mg/cat | PO q24h | |||
| FLUCONAZOLE | 5 mg/kg | PO q24h | 2 months | Pennisi et al., 2004 (0/1)^ |
| METRONIDAZOLE and SPIRAMYCIN |
MTZ : 25 mg/kg | PO q24h | In combination for 35 days | Pennisi et al., 2004 (0/1)^ |
| SPM: 150,000 UI/kg | PO q24h | |||
| ITRACONAZOLE | 50 mg/cat | PO q24h | 2 months | Pennisi et al., 2004 (0/1)^ |
Legend: MA= meglumine antimoniate; KCZ= ketaconazole; ALL= allopurinol; DOM= domperidone; Nu= nucleotides; AHCC= active hexose correlated compounds; MTZ= metronidazole; SPM= spiramycin. # 1 non-responsive cat was diagnosed with squamous cell carcinoma and the 2 others stopped therapy after few weeks because of AKI; * a single cat had to be switched to different drugs due to adverse drug reactions or lack of efficacy; ** in a single cat meglumine antimoniate was added to allopurinol when there was a clinical recurrence; ^ a single cat was switched to different drugs due lack of efficacy.
Prognosis
Some cats live with the disease for years, so the life expectancy for cats with FeL is usually good, unless concurrent conditions or complications such as CKD or neoplasia occur (Pennisi et al., 2015a; Pennisi et al., 2016; Pereira et al., 2019b; Fernandez-Gallego et al., 2020). In two studies, the median survival time in cats treated with different protocols was five and 17 months, respectively (Pennisi et al., 2016; Fernandez-Gallego et al., 2020). Based on a retrospective evaluation of 14 cases, cats died 1-72 months after diagnosis and survival time was not significantly influenced by therapy or FIV coinfection (Pennisi et al., 2016).
Prevention
In dogs, L. infantum infection is primarily prevented by reducing as much as possible exposure to sand fly bites via the use of topical pyrethroids. In recent years, vaccination has also been available, and it is an optional measure aimed to prevent the development of clinical disease (Miró et al., 2017). Almost all pyrethroids are toxic to cats, but flumethrin is licenced for cats as a collar application and in a controlled field study it decreased the incidence of L. infantum infection in cats, as had been previously seen in dogs (Brianti et al., 2014, 2017).
According to current knowledge, testing of blood donors by antibody detection and Leishmania PCR performed on EDTA blood is the only reasonable measure for preventing non-vectorial transmission to cats (Pennisi et al., 2015b).
Zoonotic risk
The Leishmania species reported in cats are of zoonotic concern, but no information is available on the risk for owners of infected cats. Vectorial transmission is considered the main way of transmission for L. infantum in humans, and dogs are considered the primary peridomestic reservoir. However, it is currently recognised that L. infantum has multiple mammalian hosts and non-canine hosts can play a primary epidemiological role in particular ecological situations (Bourdeau et al., 2020). One study performed in Iran found that owners of infected dogs had a higher risk for leishmaniosis caused by L. infantum (Gavgani et al., 2002). Currently, ownership of an infected dog is not considered a risk for the family members. However, mass preventative measures applied to dogs have a positive impact on the incidence of the human disease and on vector abundance (Silva et al., 2018; Courtenay et al., 2019).
Acknowledgement
ABCD Europe gratefully acknowledges the support of Boehringer Ingelheim (the founding sponsor of the ABCD), Virbac and IDEXX.
References
Akhtardanesh B, Kheirandish R, Shafiri I, Mohammadi A, Mostafavi A, Mahmoodi T, Ebrahimi M (2018): Low susceptibility of domestic cats to experimental leishmania infantum infection. J Vector Born Disease 55, 202-217.
Akhtardanesh B, Moeini E, Sharifi I, Saberi M, Sadeghi B, Ebrahimi M, Otranto D (2020): Leishmania infection in cats positive for immunodeficiency virus and feline leukemia virus in an endemic region of Iran. Vet Parasitol: Regional Studies and reports. https://doi.org/10.1016/j.vprsr.2020.100387
Altuzarra R, Movilla R, Roura X, Espada Y, Majo N, Novellas R (2020): Computed tomographic features of destructive granulomatous rhinitis with intracranial extension secondary to leishmaniasis in a cat. Vet radiol Ultrasound 61, E64-E68.
Asfaram S, Fakhar M, Teshnizi SH (2019): Is the cat an important reservoir host for visceral leishmaniasis? A systematic review with meta-analysis. J Venom Anim Toxins Incl Trop Dis 25:e20190012. doi: 10.1590/1678-9199-JVATITD-2019-0012. eCollection 2019.
Asgari Q, Mohammadpour I, Bozorg-Ghalati F, Motazedian MH, Kalantari M, Hosseini S (2020): Alarming: high prevalence of Leishmania infantum infection in cats from southern Iran based on molecular and serological methods. Annals of Parasitology 66, 143-146.
Attipa C, Neofytou K, Yiapanis C, Martínez-Orellana P, Baneth G, Nachum-Biala Y, Brooks-Brownlie H, Solano-Gallego L, Tasker S (2017b): Follow-up monitoring in a cat with leishmaniosis and coinfections with Hepatozoon felis and “Candidatus Mycoplasma haemominutum”. JFMS Open Rep. doi: 10.1177/2055116917740454.
Attipa C, Papasouliotis K, Solano-Gallego L, Baneth G, Nachum-Biala Y, Sarvani E, Knowles TG, Mengi S, Morris D, Helps C, Tasker S (2017a): Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit Vectors 10, 130.
Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L (2008): Canine leishmaniosis – New concepts and insights on an expanding zoonosis: part one. Trends Parasitol 24, 324-330.
Baneth G, Nachum-Biala Y, Zuberi A, Zipori-Barki N, Orshan L, Kleinerman G, Shmueli-Goldin A, Bellaiche M, Leszkowicz-Mazuz M, Salant H (2020): Leishmania infection in cats and dogs housed together in an animal shelter reveals a higher parasite load in infected dogs despite a greater seroprevalence among cats. Parasit Vectors 13, 115.
Basso MA, Marques C, Santos M, Duarte A, Pissarra H, Carreira LM, Gomes L, Valério-Bolas A, Tavares L, Santos-Gomes G (2016): Successful treatment of feline leishmaniosis using a combination of allopurinol and N-methyl-glucamine antimoniate. JFMS Open Reports 1-7.
Batista JF, Magalhães neto FDCR, Lopes KSPDP, Sato MO, Costa CHN, Mendonça IL (2020): Transmission of Leishmania infantum from cats to dogs. Rev Bras Parasitol Vet 29(4):e017820.
Baxarias M, Alvarez-Fernández M, Martínez-Orellana P, Montserrat-Sangr S, Ordeix L, Rojas S, Nachum-Biala Y, Baneth G, Solano-Gallego L (2018): Does co-infection with vector-borne pathogens play a role in clincial canine leishmaniosis? Parasit Vectors 11, 135.
Bonfante-Garrido R, Melendez E, Barroeta S (2001): Cutaneous leishmaniasis in Barquisimeto, Lara State, Venezuela. Abstract book of Worldeish 2; May 20-24, 2001; Hersonissos, Crete (Greece), p 21.
Bouchez C, Briant E, Dolon M, Navarro C, Chala V (2019): Preliminary safety study of miltefosine in healthy cats treated during 14 days. Proceedings WAAVP2019, July 7-11, 2019. Madison, WI, USA, 189.
Bourdeau P, Rowton E, Petersen C (2020): Impact of different Leishmania reservoir on sand fly transmission: perspectives from xenodiagnosis and other one health observations. Vet Parasitol 287, 109237.
Brianti E, Celi N, Napoli E, Abbate JM, Arfuso F, Gaglio G, Iatta R, Giannetto S, Gramiccia M, Otranto D (2019): Treatment and long-term follow up of a cat with leishmaniosis. Parasit Vectors 12, 121.
Brianti E, Falsone L, Napoli E, Gaglio G, Giannetto S, Pennisi MG, Priolo V, Latrofa MS, Tarallo VD, Solari Basano F, Nazzari R, Deuster K, Pollmeier M, Gulotta L, Colella V, Dantas-Torres F, Capelli G, Otranto D (2017): Prevention of feline leishmaniosis with an imidacloprid 10%/flumethrin 4.5% polymer matrix collar. Parasit Vectors 10, 334.
Brianti E, Gaglio G, Napoli E, Falsone L, Prudente C, Solari Basano F, Latrofa MS, Tarallo VD, Dantas-Torres F, Capelli G, Stanneck D, Giannetto S, Otranto D (2014): Efficacy of a slow-release imidacloprid (10%)/flumethrin (4.5%) collar for the prevention of canine leishmaniosis. Parasit Vectors 7, 327.
Can H, Döşkaya M, Özdemir HG, Şahar EA, Karakavuk M, Pektaş B, Karakuş M, Töz S, Caner A, Döşkaya AD, İz SG, Özbel Y, Gürüz Y (2016): Seroprevalence of Leishmania infection and molecular detection of Leishmania tropica and Leishmania infantum in stray cats of İzmir, Turkey. Exp Parasitol 167, 109–114.
Cardoso L, Schalling H, Persichetti F, Pennisi MG (2021): New epidemiological apsects of animal leishmaniosis in Europe: the role of vertebrate hosts other than dogs. Pathogens 10, 307.
Carneiro LA, dos Santos T, Lima L, Ramos PK, Campos MB, Silveira FT (2020): First report on feline leishmaniasis caused by Leishmania (Leishmania) amazonensis in Brazil. Vet Parasitol: Regional Studies and Rerports 19, https://doi.org/10.1016/j.vprsr.2019.100360
Costa Durao JF, Reselo E, Peleteiro MC, Correia JJ, Simoes G (1994): Primeiro caso de leishmaniose em gato domestico (Felis catus) detectado em Portugal (Concelho de Sesimbra). Nota Preliminar. Revista Portuguesa de Ciencias Veterinarias 89, 140-144.
Costa-Val AP, Coura FM, Barbieri JM, Diniz L, Sampaio A, Reis JKP, Bueno BL, Gontijo CMF (2020): Serological study of feline leishmaniasis and molecular detection of Leishmania infantum and Leishmania braziliensis in cats (Felis catus). Braz J vet Parasitol 29 https://doi.org/10.1590/S1984-29612020023
Courtenay O, Bazmani A, Parvizi P, Ready PD, Cameron MM (2019): Insecticide-impregnated dog collars reduce infantile clinical visceral leishmaniaisis under operational conditions in NW Iran: a community-wide cluster randomised trial. PLoS Negl Trop Dis 13(3):e0007193.
da Silva SM, Rabelo PF, Gontijo Nde F, Ribeiro RR, Melo MN, Ribeiro VM, Michalick MS (2010): First report of infection of Lutzomyia longipalpis by Leishmania (Leishmania) infantum from a naturally infected cat of Brazil. Vet Parasitol 174, 150-154.
da Silva SM, Ribeiro VM, Ribeiro RR, Tafuri WL, Melo MN, Michalick MS (2009): First report of vertical transmission of Leishmania (Leishmania) infantum in a naturally infected bitch from Brazil. Vet Parasitol 166, 159-162.
Dedola C, Ibba F, Manca T, Garia C, Abramo F (2015): Dermatite esfoliativa associata a leishmaniosi in un gatto. Paper presented at 2° Congresso Nazionale SIDEV, Aci Castello-Catania, Italy, 17th -19th July 2015.
de Souza AI, Barros EM, Ishikawa E, Iiha IM, Marin GR, Nunes VL (2005): Feline leishmaniasis due to Leishmania (Leishmania) amazonensis in Mato Grosso do Sul State, Brazil Vet Parasitol 128, 41-45.
Diakou A, Di Cesare A, Accettura PM, Barros L, Iorio R, Paoletti B, Frangipane di Regalbono A, Halos L, Beugnet F, Traversa D (2017): Intestinal parasites and vector-borne pathogens in stray and free-roaming cats living in continental and insular Greece. PLoS Negl Trop DIs 11(1):e0005335.
Fernandez-Gallego A, Bernabe LF, Dalmau A, Esteban-Saltiveri D, Font A, Leiva M, Ortuňez-Navarro A, Peňa MT (2020): Feline leishmaniosis: diagnosis, treatment and outcome in 16 cats. J Feline Med Surg 22, 993-1007.
Filecci I (2012): Qual’è la vostra diagnosi? Paper presented at 1st Congresso Nazionale SIDEV, Montesilvano (Pescara, Italia), 21-23 September 2012.
Franchi R, Bertazzolo W, Antoniazzi E (2019): Neoformazione rinofaringea: un caso atipico di leishmaniosi felina. Atti Congresso SCIVAC – Quali novità nella diagnosi e nella terapia della leishmaniosi. Ferrara (Italia), 5-7 aprile 2019, 89-90.
Gavgani AS, Mohite H, Edrissian GH, Mohebali M, Davies CR (2002): Domestic dog ownership in Iran is a risk factor for human infection with Leishmania infantum. Am J Trop Med Hyg 67, 511-515.
Geisweid K, Mueller R, Sauter-Louis C, Hartmann K (2012): Prognostic analytes in dogs with Leishmania infantum infection living in a non-endemic area. Vet Rec 171(16), 399.
Gonzáles E, Jiménez M, Hernández S, Martín-Martín I, Molina R (2017): Phlebotomine sand fly survey in the focus of leishmaniasis in Madrid, Spain (2012-2014): seasonal dynamics, Leishmania infantum infection rates and blood meal preferences. Parasit Vectors 10, 368.
Gradoni L (2018): A brief introduction to leishmaniasis epidemiology. In: Buschi F, Gradoni L (eds.): The leishmaniases: old neglected tropical diseases. Springer, Cham. Online ISBN 978-3-319-72386-0, pp. 1-13.
Hartmann K, Pennisi MG, Dorsch R (2020): Infectious agents in feline chronic kidney disease. What is the evidence? Advances in Small Animal Care 1, 189-206.
Hatam GR, Adnani SJ, Asgari Q, Fallah E, Motazedian MH et al (2009): First report of natural infection in cats with Leishmania infantum in Iran. Vector Borne Zoonotic Dis 10, 313-316.
Hervás J, Chacón-M De Lara F, Sánchez-Isarria MA, Pellicer S, Carrasco L, Castillo JA, Gómez-Villamandos JC (1999): Two cases of feline visceral and cutaneous leishmaniosis in Spain. J Feline Med Surg 1, 101–105.
Iatta R, Trerotoli P, Lucchese L, Natale A, Buonavoglia C, Nachum-Biala Y, Baneth G, Otranto D (2020): Validation of a new immunofluorescence antibody test for the detection of Leishmania infantum infection in cats. Parasitol Research https://doi.org/10.1007/s00436-020-06627-1
Ibba F (2009): Un caso di rinite cronica in corso di leishmaniosi felina. In: Proceedings 62nd Congresso Internazionale Multisala SCIVAC, Rimini-Italy (29th-31st May 2009), 568.
Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H; Centers for Disease Control and Prevention (CDC); National Institute of Health; HIV Medicine Association of the Infectious Diseases Society of America (2009): Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institute of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 58, 1-207.
Kirkpatrick CE, Farrell JP, Goldschmit MH (1984): Leishmania chagasi and donovani: experimental infection in domestic cats. Exp Parasitol 58, 125-131.
Leal RO, Pereira H, Cartaxeiro C, Delgado E, Peleteiro MDC, Pereira da Fonseca I (2018): Granulomatous rhinitis secondary to feline leishmaniosis: report of an unusual presentation and therapeutic complications. JFMS Open Rep 1-7. DOI: 10.1177/2055116918811374
Leiva M, Lloret A, Peña T, Roura X (2005): Therapy of ocular and visceral leishmaniasis in a cat. Vet Ophthalmol 8, 71–75.
Lombardo G, Pennisi MG, Lupo T, Migliazzo A, Caprì A, Solano-Gallego L (2012): Detection of Leishmania infantum DNA by real-time PCR in canine oral and conjunctival swabs and comparison with other diagnostic techniques. Vet Parasitol 184, 10-17.
Maia C, Gomes J, Cristóvão J, Nunes M, Martines A, Rebêlo E, Campino C (2010): Feline Leishmania infection in a canine leishmaniasis endemic region, Portugal. Vet Parsitol 174, 336-340.
Maia C, Sousa C, Ramos C, Cristóvão JM, Faísca P, Campino L (2015): First case of feline leishmaniosis caused by Leishmania infantum genotype E in a cat with a concurrent nasal squamous cell carcinoma. JFMS Open Rep 1, 2055116915593969.
Maroli M, Pennisi MG, Gramiccia M, Di Muccio T, Khouri C, Lo Giudice S et al (2007): First report of experimental Leishmania infection in Phlebotomus perniciosus fed on a cat with natural acquired leishmaniosis in Italy. Parassitologia 48, 332.
Martín-Sánchez J, Torres-Medina N, Corpas-López V, Morillas-Márquez F, Díaz-Sáez V (2020): Vertical transmission may play a greater role in the spread of Leishmania infantum in synanthropic Mus musculus rodents than previous believed. Transbound Emerg Dis 67, 1113-1118.
Mendonça IL, Batista JF, Lopes KSPDP, Magalhães Neto FDCR, Alcântara DS, Merigueti YFFB, Costa CHN (2020): Infection of Lutzomyia longipalpis in cats infected with Leishmania infantum. Vet Parasitol 280: 109058. doi: 10.1016/j.vetpar.2020.109058
Metzdorf IP, da Costa Lima MS Junior, de Fatima Cepa Matos M, de Souza Filho AF, de Souza Tsujisaki RA, Franco KG, Shapiro JT, de Almeida Borges F (2017): Molecular characterizatio of Leishmania infantum in domestic cats in a region of Brazil endemic for human and canine visceral leishmaniasis. Acta Trop 166, 121-125.
Migliazzo A, Vitale F, Calderone S, Puleio R, Binanti D, Abramo F (2014): Feline leishmaniosis: a case with a high parasitic burden. Vet Dermatol 26, 69-70.
Miró G, Petersen C, Cardoso L, Bourdeau P, Baneth G, Solano-Gallego L, Pennisi MG, Ferrer L, Oliva G (2017): Novel areas for prevention and control of canine leishmaniosis. Trends Parasitol 33, 718-730.
Mohebali M, Moradi-Asl E, Rassi Y (2018): Geographic distribution and spatial analysis of Leishmania infantum infection in domestic and wild animal reservoir hosts of zoonotic visceral leishmaniasis in Iran: a systematic review. J Vector Borne Dis 55, 173-183.
Navarro JA, Sanchez J, Peňafiel-Verdù C, Buendìa AJ, Altimira J, Vilafranca M (2010): Histopathological lesions in 15 cats with leishmaniosis. J Comp Pathol 143, 297-302.
Otranto D, Napoli E, Latrofa MS, Annoscia G, Tarallo VD, Greco G, Lorusso E, Gulotta L, Falsone L, Basano FS, Pennisi MG, Deuster K, Capelli G, Dantas-Torres F, Brianti E (2017): Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: Pathogen and vector circulation in a confined environment. Vet Parasitol 236, 144–151.
Ozon C, Marty P, Pratlong F, Breton C, Blein M, Lelièvre A, Haas P (1998): Disseminated feline leishmaniosis due to Leishmania infantum in Southern France. Vet Parasitol 75, 273-277.
Paniz Mondolfi AE, Colmenares Garmendia A, Mendoza Pérez Y, Hernández-Pereira CE, Medina C et al (2019): Autochtonous cutaneous leishmaniasis in urban domestic animals (Felis catus/Canis lupus familiaris) from central-western Venezuela. Acta Tropica 191, 252-260
Paşa S, Tetik Vardarlı A, Erol N, Karakuş M, Töz S, Atasoy A, Balcıoğlu İC, Emek Tuna G, Ermiş ÖV, Ertabaklar H, Özbel Y (2015): Detection of Leishmania major and Leishmania tropica in domestic cats in the Ege Region of Turkey. Vet Parasitol 212, 389–392.
Pennisi MG, Cardoso L, Baneth G, Bourdeau P, Koutinas A, Miró G, Oliva G, Solano-Gallego L (2015a): Leishvet update and recommendations on feline leishmaniosis. Parasit Vectors 8, 302.
Pennisi MG, Donato G, De Majo M, Mangano C, Bruno F, Castelli G, De Luca E, Pizzurro F (2019): Comorbidities and coinfections in a cat affected by leishmaniosis. Congresso SCIVAC “Quali noivtà nella diagnosi e nella terapia della leishmaniosi”. Ferrara, Italia, 5-7 aprile 2019, 98-99.
Pennisi MG, Hartmann K, Addie DD, Lutz H, Gruffydd-Jones T, Boucraut-Baralon C, Egberink H, Frymus T, Horzinek MC, Hosie MJ, Lloret A, Marsilio F, Radford AD, Thiry E, Truyen U, Möstl K, European Advisory Board on Cat Diseases (2015b): Blood transfusion in cats: ABCD guidelines for minimising risks of infectious iatrogenic complications. J Feline Med Surg 17, 588–593.
Pennisi MG, Lupo T, Malara D, Masucci M, Migliazzo A, Lombardo G (2012): Serological and molecular prevalence of Leishmania infantum infection in cats from Southern Italy [abstract]. J Feline Med Surg 14, 656-657.
Pennisi MG, Persichetti MF (2018): Feline leishmaniosis: Is the cat a small dog? Vet Parasitol 251, 131-137.
Pennisi MG, Persichetti MF, Migliazzo A, De Majo M, Iannelli NM, Vitale F (2016): Feline leishmaniosis: clinical signs and course in 14 followed up cases. In: Proceedings LXX Convegno SISVet. Palermo, Italy: (13th-16th July 2016), 166–167.
Pennisi MG, Priolo V, Ippolito D, Migliazzo A, Masucci M (2017): Evaluation of a rapid device for the serological diagnosis of Leishmania infantum infection in cats. Proceedings WorldLeish 6, 16th-20th May 2017. Toledo, Spain, C1003.
Pennisi MG, Venza M, Reale S, Vitale F, Lo Giudice S (2004): Case report of leishmaniasis in four cats. Vet Res Comm 28, 363-366.
Pereira A, Cristóvão JM, Vilhena H, Martins A, Cachola P, Henriques J, Coimbra M, Catarino A, Lestinova T, Spitzova T, Volf P, Campino L, Maia C (2019a): Antibody response to Phlebotomus perniciosus saliva in cats naturally exposed to phlebotomine sand flies is positively associated with Leishmania infection. Parasit Vectors 12, 128.
Pereira A, Valente J, Parreira R, Cristovão JM, Azinheira S, Campino L, Maia C (2019b): An unusual case of feline leishmaniosis with involvement of the mammary glands. Top Companion Anim Med 37, 100356. doi: 10.1016/j.tcam.2019.100356
Perillo L, Pennisi MG, Solano-Gallego L, Lupo T, Migliazzo A, Mazzullo G (2013): Leishmania infantum PCR positive lymph node aspirates: cytological patterns in cats. Proceedings International SCIVAC Congress – Canine leishmaniosis and other vector-borne diseases: our current state of knowledge. March 8th-10th 2013, Pisa-Italy, 144-145.
Persichetti M-F, Solano-Gallego L, Serrano L, Altet L, Reale S, Masucci M, Pennisi M-G (2016): Detection of vector-borne pathogens in cats and their ectoparasites in southern Italy. Parasit Vectors 9, 247.
Persichetti MF, Solano-Gallego L, Vullo A, Masucci M, Marty P, Delaunay P, Vitale F, Pennisi MG (2017): Diagnostic performance of ELISA, IFAT and Western blot for the detection of anti-Leishmania infantum antibodies in cats using a Bayesian analysis without a gold standard. Parasit Vectors 10, 119.
Pimenta P, Alves-Pimenta S, Barros J, Barbosa P, Rodrigues A, Pereira MJ, Maltez L, Gama A, Cristóvão JM, Campino L, Maia C, Cardoso L (2015): Feline leishmaniosis in Portugal: 3 cases (year 2014). Vet Parasitol Reg Stud Reports 1–2, 65–69. doi:10.1016/j.vprsr.2016.02.003.
Pocholle E, Reyes-Gomez E, Giacomo A, Delaunay P, Hasseine L, Marty P (2012): Un cas de leishmaniose féline disséminée dans le sud de la France. Le chat (Felis catus), réservoir potential de Leishmania infantum. Parasite 19, 77-80.
Poli A, Abramo F, Barsotti P, Leva S, Gramiccia M, Ludovisi A, Mancianti F (2002): Feline leishmaniosis due to Leishmania infantum in Italy. Vet Parasitol 106, 181–191.
Priolo V, Martínez Orellana P, Pennisi MG, Masucci M, Prandi D, Ippolito D, Bruno F, Castelli G, Solano-Gallego L (2019): Leishmania infantum-specific IFNγ production in stimulated blood from cats in areas where canine leishmaniosis is endemic. Parasit Vectors 26, 133.
Puleio R, Tamburello A, Lupo T, Migliazzo A, Loria GR, Pennisi MG (2011): Aspetti istopatologici, immunoistochimici e molecolari in quattro casi di leishmaniosi felina. Proceedings of the 8th National Congress of the Italian Society of Veterinary Pathologists (AIPVet); 2011 Jun 15-17; Padova, Italia, p 87.
Richter M, Schaarschmidt-Kiener D, Krudewig C (2014): Ocular signs, diagnosis and long-term treatment with allopurinol in a cat with leishmaniasis. Schweiz Arch Tierheilkd 156, 289–294.
Rivas AK, Alcover M, Martínez-Orellana P, Montserrat-Sangrà S, Nachum-Biala Y, Bardagí M, Fisa R, Riera C, Baneth G, Solano-Gallego L (2018): Clinical and diagnostic aspects of feline cutaneous leishmaniosis in Venezuela. Parasit Vectors 11, 141.
Rougeron V, Catzeflis F, Hide M, De Meeûs T, Bañuls AL (2011): First clinical case of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis in a domestic cat from French Guiana. Vet Parasitol 181, 325-328.
Roura X, Cortadellas O, Day MJ, Benali SL, CLWG, Zatelli A (2020): Canine leishmaniosis and kidney disease: Q&A for an overall management in clinical practice. J Small Anim Pract. doi: 10.1111/jsap.13237
Rüfenacht S, Sager H, Mueller N, Schaerer V, Heier A, Welle MM et al (2005): Two cases of feline leishmaniasis in Switzerland. Vet Rec 156, 542-545.
Sanches A, Pereira AG, Carvalho JP (2011): Un caso de leishmaniose felina. Vet Med 63, 29–30.
Savioli G, Archer J, Brinti E, Benelli G, Schnyder M, Iatta R, Otranto D, Cantacessi C (2021): Serum amyloid A levels and alpha 2 and gamma globulins on serum protein electrophoresis in cats exposed to and infected with Leishmania infantum. Parasites Vectors 14, 217.
Schubach TM, Figueredo FB, Pereira SA, Madeira MF, Santos IB, Andrade MV (2004): American cutaneous leishmaniasis in two cats from Rio de Janeiro, Brazil: first report of natural infection with Leishmania (Viannia) braziliensis. Transaction of the Royal Society of Tropical Medicine and Hygiene 98, 165-167.
Silva RAE, Andrade AJ, Quint BB, Raffoul GES, Werneck GL, Rangel EF, Romero GAS (2018): Effectiveness of dog collars impregnated with 4% deltamethrin in controlling visceral leishmaniasis in Lutzomyia longipalpis (Diptera: Psychodidade: Phlebotominae) populations. Mem Inst Oswaldo cruz 113(5):e170377.
Simoes-Mattos L, Mattos MR, Teixeira MJ, Oliveira-Lima JW, Bevilacqua CM, Prata-Junior RC et al (2005): The susceptibility of domestic cats (Felis catus) to experimental infection with Leishmania braziliensis. Vet Parasitol 127, 199-208.
Solano-Gallego L, Koutinas A, Miró G, Cardoso L, Pennisi MG, Ferrer L et al (2009): Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol 165, 1-18.
Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G (2011): LeishVet guidelines for the pratical management of canine leishmaniosis. Parasit Vectors 4, 86.
Toepp AJ, Schaut RG, Scott BD, Mathur D, Berens AJ, Petersen CA (2017): Leishmania incidence and prevalence in U.S. hunting dogs maintained via vertical transmission. Vet Parasitol Reg Stud Reports 10, 75-81.
Trainor KE, Porter BF, Logan KS, Hoffman RJ, Snowden KF (2010): Eight cases of feline cutaneous leishmaniasis in Texas. Vet Pathol 47, 1076-1081.
Urbani L, Tirolo A, Salvatore D, Tumbarello M, Segatore S, Battilani M, Balboni A, Dondi F (2020): Serological, molecular and clinicopathological findings associated with Leishmania infantum infection in cast in Northern Italy. J Feline Med Surg 22, 935-943.
Verneuil M (2013): Ocular leishmaniasis in a cat: case report. J Fr Ophtalmol 36(4):e67-72.
Vides JP, Schwardt TF, Sobrinho LS, Marinho M, Laurenti MD, Biondo AW et al (2011): Leishmania chagasi infection in cats with dermatologic lesions from an endemic area of visceral leishmaniosis in Brazil. Vet Parasitol 178, 22-28.

